| |
|
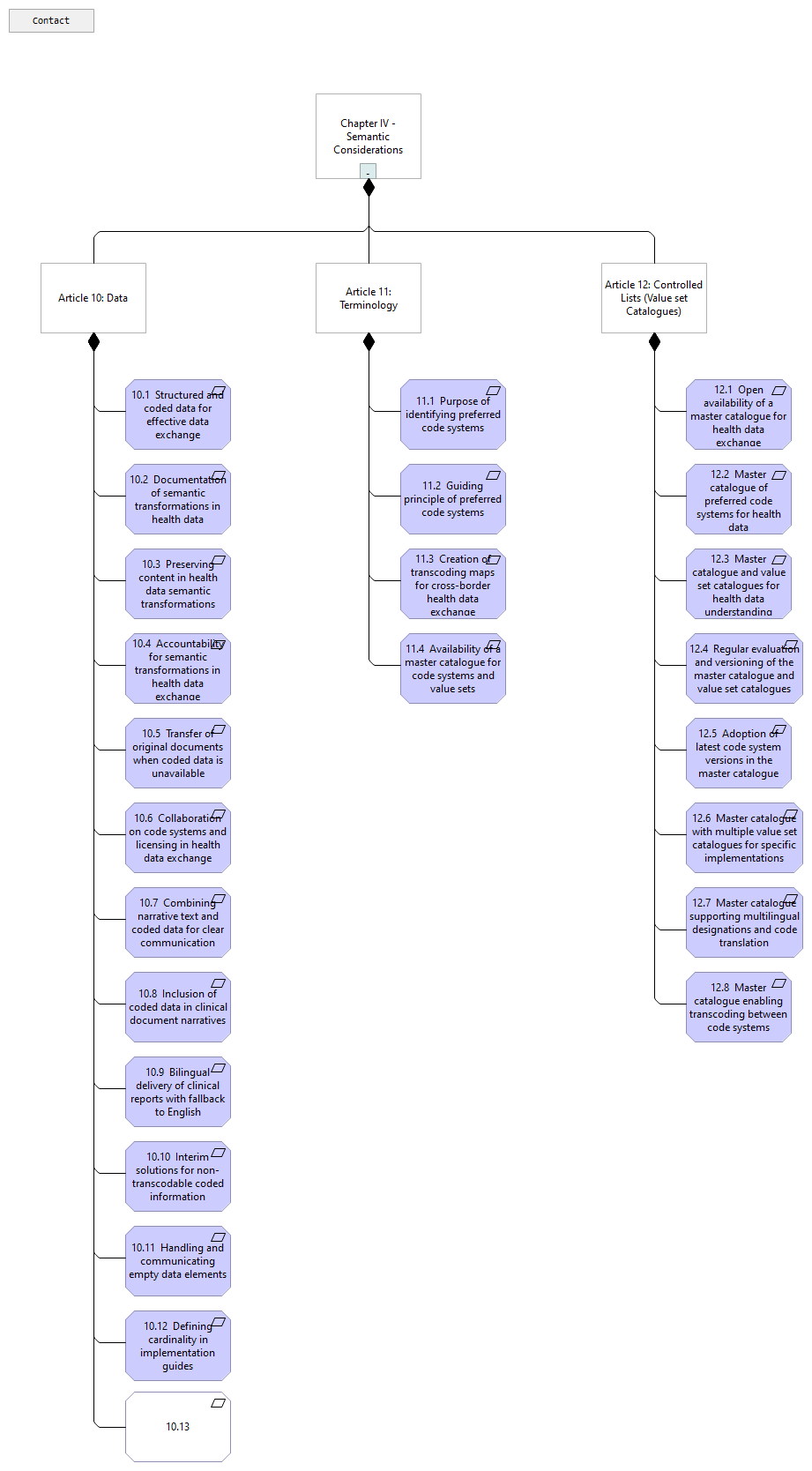
Chapter IV - Semantic Considerations |
Article 10: Data |
| |
|
Chapter IV - Semantic Considerations |
Article 11: Terminology |
| |
|
Chapter IV - Semantic Considerations |
Article 12: Controlled Lists (Value set Catalogues) |
| |
|
Article 10: Data |
10.3 Preserving content in health data semantic transformations |
| |
|
Article 10: Data |
10.13 |
| |
|
Article 10: Data |
10.9 Bilingual delivery of clinical reports with fallback to English |
| |
|
Article 10: Data |
10.2 Documentation of semantic transformations in health data |
| |
|
Article 10: Data |
10.8 Inclusion of coded data in clinical document narratives |
| |
|
Article 10: Data |
10.6 Collaboration on code systems and licensing in health data exchange |
| |
|
Article 10: Data |
10.12 Defining cardinality in implementation guides |
| |
|
Article 10: Data |
10.11 Handling and communicating empty data elements |
| |
|
Article 10: Data |
10.7 Combining narrative text and coded data for clear communication |
| |
|
Article 10: Data |
10.1 Structured and coded data for effective data exchange |
| |
|
Article 10: Data |
10.4 Accountability for semantic transformations in health data exchange |
| |
|
Article 10: Data |
10.5 Transfer of original documents when coded data is unavailable |
| |
|
Article 10: Data |
10.10 Interim solutions for non-transcodable coded information |
| |
|
Article 11: Terminology |
11.4 Availability of a master catalogue for code systems and value sets |
| |
|
Article 11: Terminology |
11.3 Creation of transcoding maps for cross-border health data exchange |
| |
|
Article 11: Terminology |
11.2 Guiding principle of preferred code systems |
| |
|
Article 11: Terminology |
11.1 Purpose of identifying preferred code systems |
| |
|
Article 12: Controlled Lists (Value set Catalogues) |
12.5 Adoption of latest code system versions in the master catalogue |
| |
|
Article 12: Controlled Lists (Value set Catalogues) |
12.8 Master catalogue enabling transcoding between code systems |
| |
|
Article 12: Controlled Lists (Value set Catalogues) |
12.7 Master catalogue supporting multilingual designations and code translation |
| |
|
Article 12: Controlled Lists (Value set Catalogues) |
12.1 Open availability of a master catalogue for health data exchange |
| |
|
Article 12: Controlled Lists (Value set Catalogues) |
12.4 Regular evaluation and versioning of the master catalogue and value set catalogues |
| |
|
Article 12: Controlled Lists (Value set Catalogues) |
12.2 Master catalogue of preferred code systems for health data |
| |
|
Article 12: Controlled Lists (Value set Catalogues) |
12.6 Master catalogue with multiple value set catalogues for specific implementations |
| |
|
Article 12: Controlled Lists (Value set Catalogues) |
12.3 Master catalogue and value set catalogues for health data understanding |